When you work in the mobility medical device industry, whether you build motorized wheelchairs, scooters, or other assistive devices, you know that getting products to people who need them isn’t just about shipping orders. It’s about making something that works every time, meets strict standards, and gets out the door fast enough to keep up with demand.
The challenges are real when it comes to regulated, complex assembly. Maybe you’ve spent late nights checking paperwork or wrestling with unexpected slowdowns on the assembly line. Maybe you’ve worried about the next regulatory audit or wondered how you’ll manage a sudden jump in orders. These moments shape the work we do, and the choices we make every day.
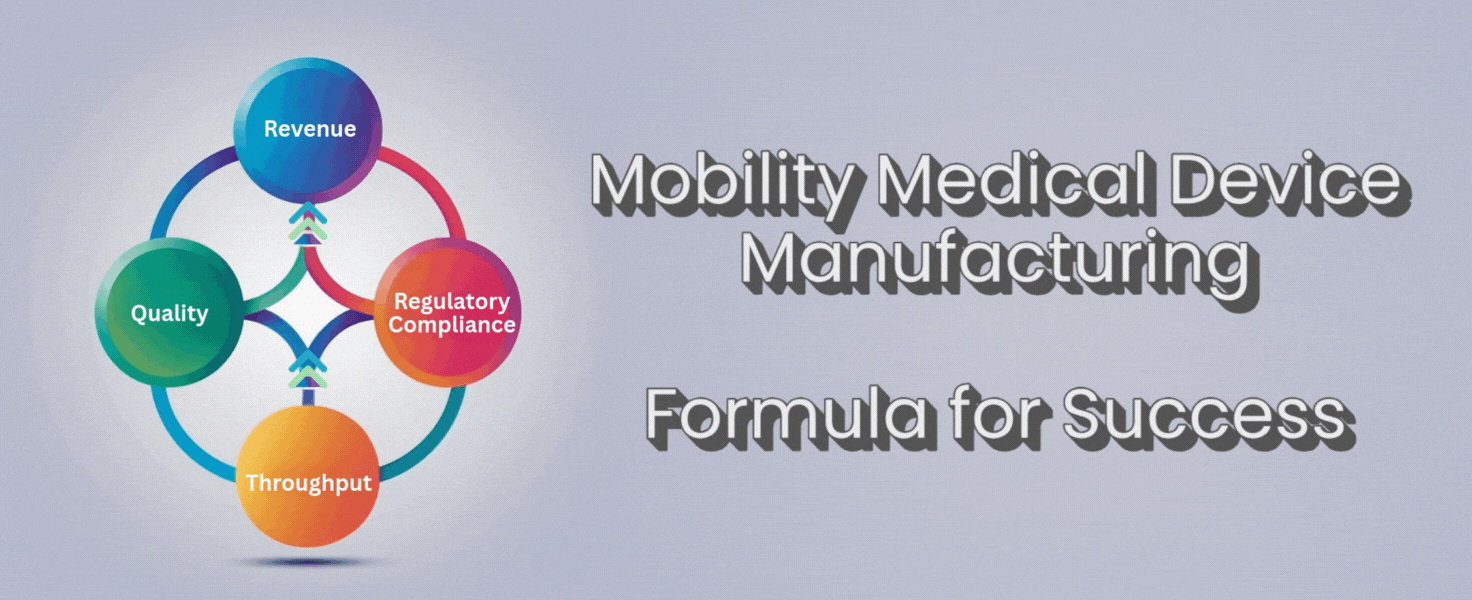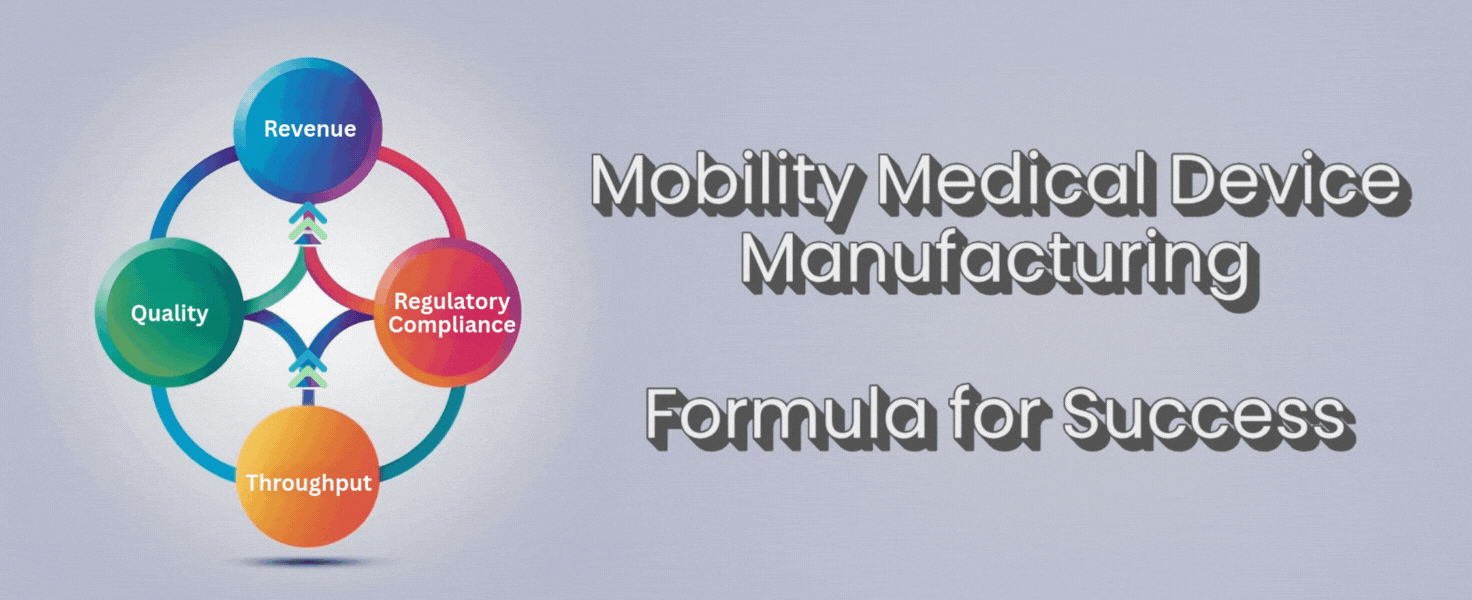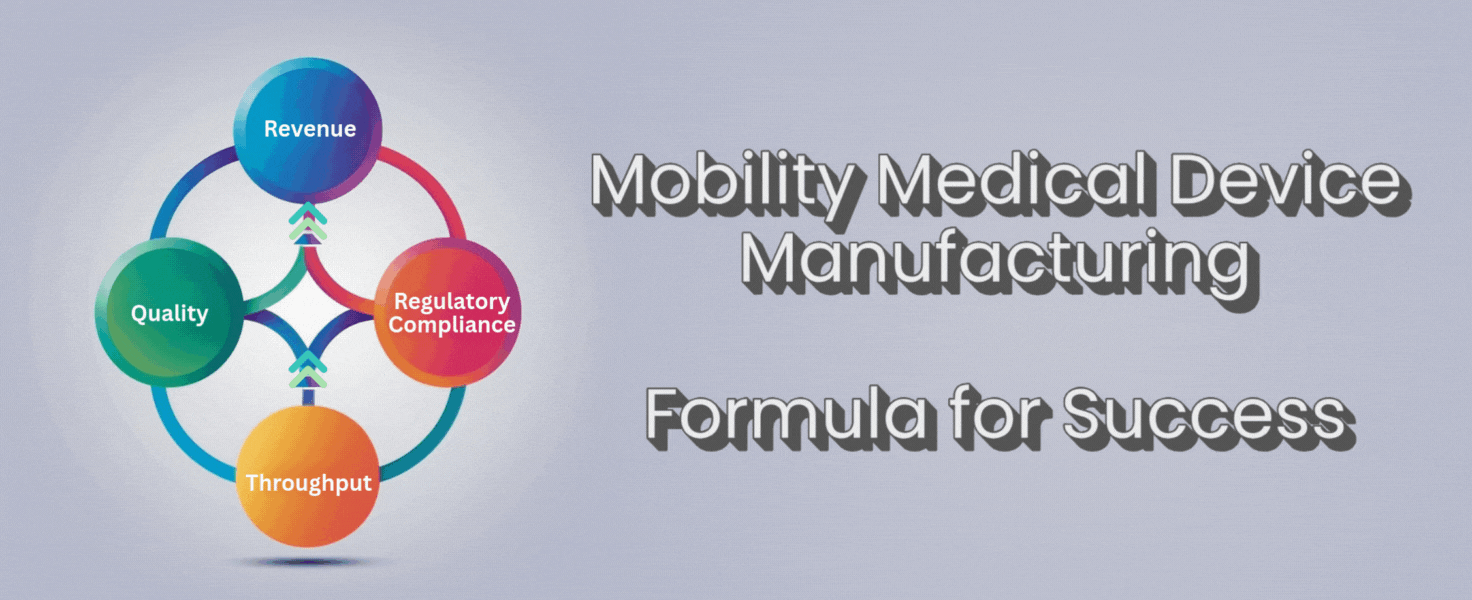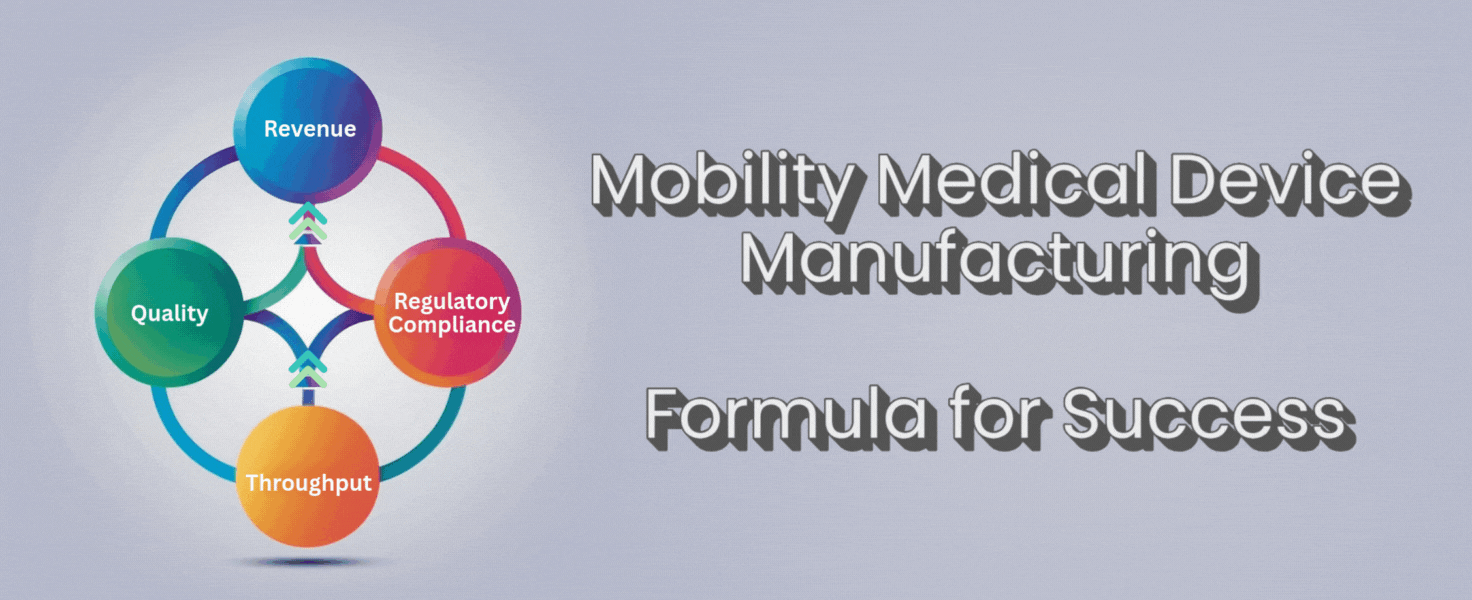
There’s a simple formula that can help you cut through the noise:
Throughput + Quality + Regulatory Compliance = Revenue
Let’s talk about what that means for you, and how you can use it to steer your business toward steady growth and fewer headaches. Matrix Automation, a solution provider for complex medical device manufacturers since 1983, offers a practical approach to each step.
Throughput: Getting Products Out the Door
Throughput is a straightforward idea. It’s about how many finished devices you ship in a given time. The more you ship, the more people you help, and the more money you make. But throughput isn’t just about speed. It’s about reliable speed.
If you’re building motorized wheelchairs or scooters, your assembly line is complex. Parts need to come together in the right order. Each device might have custom features. One misstep can slow everything. Sometimes, tracking what’s happening in real time is tough.
A smart way to improve throughput is to digitize your assembly process. Using digital records and instructions, you can guide workers through each step, spot bottlenecks, and fix problems fast. It also helps you spot trends—maybe a certain part always causes delays, or a step needs clearer instructions. Digital tools make it easier to adjust on the fly.
Matrix Automation has helped manufacturers streamline their assembly lines by digitizing complex processes. Matrix engineers understand that every minute saved is a minute you can use to help someone get moving again. These manufacturing systems make it simpler to see what’s holding things up and how to fix it. Explore our case study with Invacare.
Quality: Making Products People Can Trust
Quality isn’t optional in medical device manufacturing. Your customers rely on your products for freedom and independence. If a controller doesn’t work properly, a motor on a wheelchair fails, a scooter’s brakes don’t work, the consequences can be serious. You want every device leaving your factory to work the way it should.
Quality comes down to error-proofing. That means building systems where mistakes are caught before they become problems. You might use sequenced testing to check if parts are functioning correctly, and software to track each step. You might check every final product before shipping. Bottom line: in complex assembly, real quality starts with human-centered design.
Matrix Automation uses proven error-proofing methods for Quality At The Source (QATS). The manufacturing solution helps you catch mistakes as they happen, so you don’t find them after it’s too late. They build in checks for human actions and for automated steps. The goal is to make every operator an expert through digital support—it’s to make their jobs easier, so they can be confident in what they build. Learn how Matrix improved quality and optimized operations at Pride Mobility.
Quality isn’t just about preventing errors. It’s also about learning from them. If something goes wrong, having a clear record helps you figure out why and fix it for good. That’s where digital records and analysis come in.
Regulatory Compliance: Navigating the Rules
If you’re making medical devices, you know compliance with regulations like FDA 21 CFR 820 or ISO 13485 is critical. These rules aren’t just boxes to check. They set standards for safety, reliability, and traceability.
Keeping up with regulations can feel overwhelming. There’s paperwork, recordkeeping, and audits. Sometimes, rules change, and you need to adapt quickly.
One way to make compliance easier is to automate with Electronic Device History Records (eDHR). This means every device has a digital thread—a complete, searchable record from start to finish. If an inspector comes in or there’s a problem with a specific product, you can pull up exactly what you need. If you need to recall, you know which devices are affected.
Matrix Automation has been helping manufacturers stay on top of these requirements since 1983. Our manufacturing systems make compliance less of a burden and more of a routine part of your process. You don’t have to worry about missing signatures or losing paperwork. It’s all in the system, ready when you need it. Get more insights in our blog about Compliance and Medical Manufacturing.
Flexibility: Adapting to Change
Nothing stays the same for long. Demand goes up and down. Products change. Workers come and go. Your systems need to be flexible, not rigid.
Matrix Automation designs processes that adapt with you. If you need to update a product, add a new feature, or train new staff, our systems can handle it. Flexibility means less downtime, fewer surprises, and better morale on the floor. When your process can change, your business can grow.
Putting It All Together
Throughput, quality, and compliance aren’t separate boxes to check. They work together. If you push throughput but lose quality, you’ll lose trust and face more returns. If you focus on quality but ignore throughput, you’ll fall behind competitors and lose orders. If you forget compliance, you risk fines or worse.
When you get these three elements working together, revenue follows. More devices shipped for customers, fewer returns or repairs, and no lost time scrambling for compliance. You get to spend your time growing the business, not fighting fires.
Matrix Automation brings all three together with practical solutions tailored to mobility medical device makers. Our solutions are not about buzzwords or flashy technology. They’re about helping you build what works, day in and day out.
Getting Started
If you’re wondering where to begin, take stock of where you stand in each area. Are you hitting your throughput targets? Are defects causing delays or warranty claims? Is your compliance process routine or stressful? Look at where you have pain points.
Sometimes, a small change makes a big difference. Digitizing records, updating instructions, or automating a check can free up time and reduce stress. You don’t have to flip everything overnight.
Matrix Automation is a resource you can lean on. Since 1983, we’ve worked with manufacturers like you to build systems that last. We know the roadblocks you face because we’ve helped others overcome them.
Working Smarter
Success in mobility medical device manufacturing isn’t about running faster or working harder. It’s about working smarter—making sure throughput, quality, and compliance are all part of your daily routine. When those parts are solid, revenue follows. You help more people, build a stronger business, and spend less time worrying.
If you’re ready to make a change, or just want to talk through your options, reach out and start the conversation. There’s no magic formula. Just a simple and proven one:
Throughput + Quality + Regulatory Compliance = Revenue.
Ready to implement the formula for success? Contact us today or learn more about our solutions for mobility medical device manufacturers.